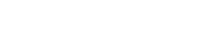Summary
The LionHeartTM LVAS System
The implantable LionHeartTM subsystems cumulatively weigh 1.45 kilogrammes. The system is modular in design to facilitate implantation and replacement of components over time.
The electrically powered blood pump features a motor, a pusher plant mechanism, a smooth blood sac, and two tilting disk valves for unidirectional flow.
The motor controller and internal coil control the operation of the blood pump. External power is received transcutaneously by the internal coil and sent to the motor controller and blood pump for continuous operation. Internal power is delivered by the motor controller’s rechargeable batteries and allows the recipient to function free of the external power source for up to 20 minutes.
The Compliance chamber and access port serve as a gas-volume accumulator, providing gas to evacuated chambers of the blood pump during its operation. Gas is replenished in the compliance chamber with a simple refill procedure through the access port approximately every four weeks.
The power transmitter and coil transfer power across the intact skin. Power is derived from an external battery pack.
SKF roller screw helps keep the beat
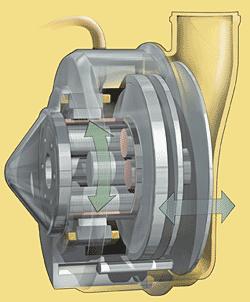
A key element in ensuring the reliability of the LionHeartTM LVAS blood pump assembly is a custom-made SKF planetary roller screw, a linear motion actuation component consisting of a threaded screw shaft, a threaded nut and a set of rollers that engage both the nut and shaft. There is an internal timing gear mechanism in the nut that ensures the rollers continuous rotation without skidding or slipping. The 8mm x 4mm roller screw is designed to facilitate precise operation of the pump’s pusher plate. It is constructed of stainless steel to eliminate any potential of corrosion caused by the moist human environment.
As the rotor spins in a clockwise and counterclockwise direction, the roller screw drives the pusher plate back and forth within the blood pump housing to compress and empty a blood sac that has been passively filled with blood from the left ventricle through a one-way tilting disk valve. The blood pump works continuously in a “fill-to-empty” mode to pump blood.
While most LVAS components can be accessed fairly easily, the implanted pump is located in an area that can be reached only through an invasive surgical procedure, making reliability of the roller screw even more critical.
With the assistance of the LionHeart, the natural heart beats 50 million times a year. The roller screw needs to match that rate with extreme accuracy. The screw makes 4.25 revolutions as it drives the pusher plate forward, and 4.25 revolutions as it comes back in, for a total of 8.5 revolutions per cycle, or 425 million cycles per year. Over four years, the estimated life of the current Lionheart LVAS device components, the roller screw cycles 1.7 billion times.
Exhaustion from brushing your teeth? That’s a reality for millions of patients with end-stage congestive heart failure. Now these patients have the prospect of a new lease on life, through the recently developed LionHeartTM Left Ventricular Assist System.Imagine being afraid to fall asleep for fear you might never wake up. One person in Germany – a man with end-stage congestive heart failure – lived with this fear until his life was improved by a medical miracle called the LionHeartTM Left Ventricular Assist System (LVAS).
Commonly referred to as an artificial heart, the LionHeart is actually a system that works with the patient’s natural heart, pumping blood and managing its flow in and out of the heart. The system was developed through a seven-year collaborative effort between Pennsylvania State University’s (Penn State) Medical School at the Milton S. Hershey Medical Center in Hershey, Pennsylvania, USA and Arrow International, a worldwide manufacturer and innovator of medical devices headquartered in Reading, Pennsylvania.
More than 15 million people worldwide suffer from congestive heart failure, a degenerative illness caused by the inability of the heart to maintain adequate blood circulation in the peripheral tissues and the lungs. As it progresses, patients become weaker and less able to manage daily functions. Patients in the final stages live a bed-to-chair existence, experiencing shortness of breath and extreme weakness. Often, the simple act of brushing teeth can result in complete exhaustion. These patients look forward to little more than multiple medications and multiple hospitalisations, with no hope of stopping the progress of the disease.
The LionHeart, an electrically driven assist pump implanted in the patient’s abdomen, provides a therapeutic option for patients who do not qualify for a heart transplant and are no longer responding to medications. In the new system, a motor-driven pump takes blood from the heart’s weakened left ventricle and pumps it to the aorta, the body’s largest artery.
The first fully implantable LVAS in clinical use, the LionHeart requires no lines or cables going through the skin to power the device. Instead, energy for the system is delivered across the skin to a coil surgically implanted under the skin. An external coil worn by the patient connects to a portable power source. This method eliminates the potential for infection involved with external lines, and allows patients greater mobility.
Three decades of research
Essential research in the area of sustained mechanical circulatory support that helped to define and develop the LionHeart has been ongoing at Penn State for the past 30 years. Gus Rosenberg, Ph.D., Penn State’s chief of the division of artificial organs, and Jane E. Fetter, a professor of surgery at the university, directed the team who developed the advanced prototype that evolved into the LionHeart. Their work was greatly influenced by Penn State’s William Pierce, M.D., and Walter Pae, M.D., leaders in the field of artificial heart research.
“Our primary concern has always been reliability,” notes Rosenberg. “A natural heart beats 50 million times a year. The implanted device must deliver absolute precision in facilitating blood flow for every one of those beats.”
Arrow International entered the picture in the early 1990s. A medical device manufacturer with broad experience in mechanical design and production, Arrow helped refine the functionality of the LionHeart, develop manufacturing capabilities and advance commercialisation.
Jim Thompson, Arrow’s manager of strategic planning and artificial heart development, explains that a major collaboration with Penn State involved making the LionHeart a completely modular system. “Initially, all of the subsystems were hardwired together,” he notes. “We assisted the Penn State team in making the system completely modular, to enable replacement or upgrading of individual subsystems as the need arises after implantation.”
Arrow also worked on developing other aspects of the LionHeart, including surgical accessories needed for implantation. The company created a single procedure kit that includes the LionHeart components, specially designed surgical tools, and every component needed by both the doctor and patient.
A chance for a better life
The first human implant of the LionHeart occurred at the Heart and Diabetes Centre in Bad Oeynhausen, Germany, on October 26, 1999. The patient was a 65-year-old retired engineer. His wife was a nurse. This, in addition to the patient’s meeting the medical qualifications for implantation of the LionHeart, made him an ideal candidate. The patient is now well into his second year of life with the Lionheart – a device he affectionately refers to as his “best friend.”
Another patient, who will soon celebrate his one-year anniversary with the LionHeart, has begun to resume some of the activities of a normal life – including an occasional spin on his bike. A third patient, the one who previously found it difficult to sleep, says the greatest gift the device has offered him is peace of mind.
As of the end of 2000, 10 patients have received the LionHeart as part of the ongoing European clinical investigation sponsored by Arrow and Penn State. In addition to Bad Oeynhausen, clinical sites for the trials include the Hôpital de la Pitié in Paris, France; the University of Vienna Medical Centre in Vienna, Austria; and the Berlin Heart Institute in Berlin, Germany. In February 2001, the United States Food and Drug Administration approved human clinical trials of the LionHeart in the US. Patients will be enrolled in the study throughout 2001.
Refinements
Even as clinical trials progress, Arrow and Penn State are working on refinements to the LionHeart, including a smaller, longer-lasting battery, miniaturisation of components, and extended operational reliability. “We’d like to extend the expected life of the system to at least 10 years,” notes Rosenberg. “Right now, with the exception of batteries, we feel that we may count on system reliability for up to four years before components need to be replaced.”
The life-changing impact of the LionHeart is a source of satisfaction for everyone involved on the Penn State and Arrow development teams. “It’s amazing how quickly patients begin to feel better,” notes Rosenberg. “Congestive heart failure patients are so frail. Their skin is grey, and their bodies just can’t function. Just a few days after the LionHeart is implanted, we see a dramatic difference.”
Joyce Williams
a business journalist based in Kimberton,
Pennsylvania, USA
photo Arrow International
illustration Peter Forslund